The word Diamond comes from the Greek word Adamas, which means indestructible. It is the only gem known to man that is made of a single element, Carbon, besides graphite. Diamond is completely made of Carbon atoms (Chemical Composition – C) crystallized in a cubic (isometric) arrangement.
The word Diamond comes from the Greek word Adamas, which means indestructible. It is the only gem known to man that is made of a single element, Carbon, besides graphite. Diamond is completely made of Carbon atoms (Chemical Composition – C) crystallized in a cubic (isometric) arrangement.
How and where are diamonds formed?
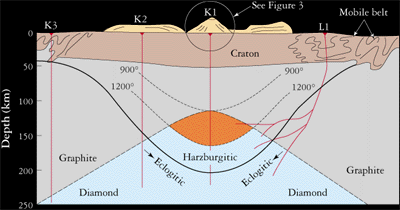
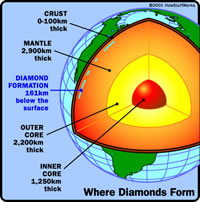 Diamonds form between 120-200 kilometers or 75-120 miles below the earth’s surface. According to geologists the first delivery of diamonds was somewhere around 2.5 billion years ago and the most recent was 45 million years ago. According to science, the carbon that makes diamonds comes from the melting of pre-existing rocks in the Earth’s upper mantle. There is an abundance of carbon atoms in the mantle.
Diamonds form between 120-200 kilometers or 75-120 miles below the earth’s surface. According to geologists the first delivery of diamonds was somewhere around 2.5 billion years ago and the most recent was 45 million years ago. According to science, the carbon that makes diamonds comes from the melting of pre-existing rocks in the Earth’s upper mantle. There is an abundance of carbon atoms in the mantle.
Temperature changes in the upper mantle forces the carbon atoms to go deeper where it melts and finally becomes new rock, when the temperature reduces. If other conditions like pressure and chemistry are right then the carbon atoms in the melting crystal rock bond to build diamond crystals.
There is no guarantee that these carbon atoms will turn into diamonds. If the temperature rises or the pressure drops then the diamond crystals may melt partially or totally dissolve. Even if they do form, it takes thousands of years for those diamonds to come anywhere near the surface.
How do diamonds get to the surface?
Diamonds ascend to the Earth’s surface in rare molten rock, or magma that originates at great depths. Carrying diamonds and other samples from Earth’s mantle, this magma rises and erupts in small but violent volcanoes. Just beneath such volcanoes is a carrot-shaped “pipe” filled with volcanic rock, mantle fragments, and some embedded diamonds. The rock is called kimberlite after the city of Kimberley, South Africa, where the pipes were first discovered in the 1870s. Another rock that provides diamonds is lamproite.
The volcano that carries diamond to the surface emanates from deep cracks and fissures called dikes. It develops its carrot shape near the surface, when gases separate from the magma, perhaps accompanied by the boiling of ground water, and a violent supersonic eruption follows. The volcanic cone formed above the kimberlite pipe is very small in comparison with volcanoes like Mount St. Helens, but the magma originates at depths at least 3 times as great. These deep roots enable kimberlite to tap the source of diamonds. Magmas are the elevators that bring diamonds to Earth’s surface.
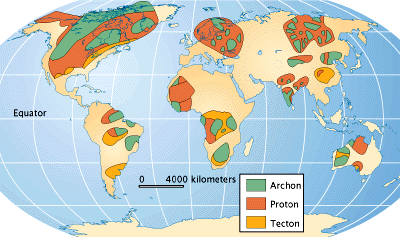
The search for diamonds has determined that most are derived from kimberlite pipes in the oldest, nuclear portions of the continents, where the basement rocks are older than 1.5 billion years. The oldest parts of continents are called cratons, and can be divided into two terranes: Archean-age archons, which are older than 2,500 million years, and Proterozoic-age protons, which are 1,600 — 2,500 million years old. The distribution of these terranes is shown on the map.
The complex volcanic magmas that solidify into kimberlite and lamproite are not the source of diamonds, only the elevators that bring them with other minerals and mantle rocks to Earth’s surface. Although rising from much greater depths than other magmas, these pipes and volcanic cones are relatively small and rare, but they erupt in extraordinary supersonic explosions.
Kimberlite and lamproite are similar mixtures of rock material. Their important components include fragments of rock from Earth’s mantle, large crystals, and the crystallized magma that glues the mixture together. The magmas are very rich in magnesium and volatile compounds such as water and carbon dioxide. As the volatiles dissolved in the magma change to gas near Earth’s surface, explosive eruptions create the characteristic carrot- or bowl-shaped pipes.
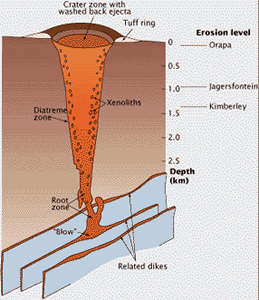 Kimberlite magma rises through Earth’s crust in networks of cracks or dikes. The pipes only form near Earth’s surface. This cross-section of a kimberlite pipe shows the carrot-shaped profile produced by explosive eruption. The root zone starts in fissures, where gases are released from the rising magma and drive the eruption; they blow out the fragment-laden kimberlite to form the volcano’s tuff ring and fill the pipe.
Kimberlite magma rises through Earth’s crust in networks of cracks or dikes. The pipes only form near Earth’s surface. This cross-section of a kimberlite pipe shows the carrot-shaped profile produced by explosive eruption. The root zone starts in fissures, where gases are released from the rising magma and drive the eruption; they blow out the fragment-laden kimberlite to form the volcano’s tuff ring and fill the pipe.
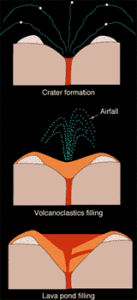 These drawings illustrate the formation and filling of the typical champagne-glass shape of a lamproite pipe. The initial stage of the eruption, powered by gases either from the lamproite magma or from boiling ground water, corrodes the hosting rock to form the champagne-glass shape (top). The eruption then produces particles of ash, lapilli, and pumice that partially fill the crater and form a tuff ring (middle). Finally, the crater fills with a lava pond from the degassed lamproite magma (bottom).
These drawings illustrate the formation and filling of the typical champagne-glass shape of a lamproite pipe. The initial stage of the eruption, powered by gases either from the lamproite magma or from boiling ground water, corrodes the hosting rock to form the champagne-glass shape (top). The eruption then produces particles of ash, lapilli, and pumice that partially fill the crater and form a tuff ring (middle). Finally, the crater fills with a lava pond from the degassed lamproite magma (bottom).
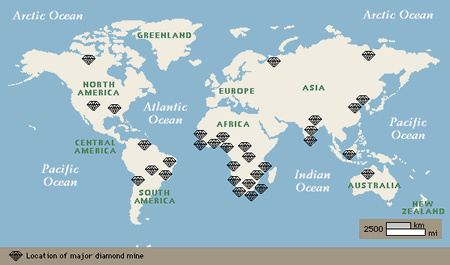
Today diamonds are mined in about 25 countries, on every continent but Europe and Antarctica. However, only a few diamond deposits were known until the 20th century, when scientific understanding and technology extended diamond exploration and mining around the globe. For 1,000 years, starting in roughly the 4th century BC, India was the only source of diamonds. In 1725, important sources were discovered in Brazil, and in the 1870s major finds in South Africa marked a dramatic increase in the diamond supply. Additional major producers now include several African countries, Siberian Russia, and Australia.
It is a modern misconception that the world’s diamonds come primarily from South Africa. However, diamonds are a world-wide resource. The common characteristic of primary diamond deposits is the ancient terrain that hosts the kimberlite and lamproite pipes that bring diamonds to Earth’s surface.
The map above shows both the major deposits and the ancient bedrock, both the 2,500-million-year-old archons and less productive 1,600 to 2,500-million-year-old protons, that contain the diamond pipes. The diamonds in secondary deposits have been moved by erosion away from the pipes.
Diamond Deposits
Geologic processes create two basic types of diamond deposits, referred to as primary and secondary sources. Primary sources are the kimberlite and lamproite pipes that raise diamonds from Earth’s mantle, where they originate. Secondary sources, created by erosion, include such deposits as surface scatterings around a pipe, concentrations in river channels, and fluxes from rivers moved by wave action along ocean coasts, past and present. Mining of these deposits depends upon sufficient concentration and quality of diamonds.
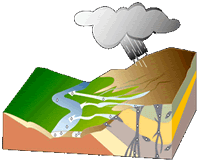 The diagram here shows the trail of diamonds left by geological processes.
The diagram here shows the trail of diamonds left by geological processes.
The primary deposits, or diamond pipes, are the vertical portion. The flared top of the pipes can yield substantial quantities of diamonds, but following the narrowing pipe downward eventually becomes unprofitable. Note how erosion of the landscape moves surface minerals, including the diamonds, from the pipes down hills, streams, and rivers to their ultimate destination, the ocean.
Because diamonds are dense they concentrate at the bottom zones of moving sand and gravel. These secondary deposits are eluvial (above a pipe), colluvial (adjacent to a pipe), alluvial (stream and river transported) and marine (along beaches that can wind up onshore or offshore with changing sea level). Secondary deposits may be found far from current means of transport, in the fossilized channels of now-vanished rivers or under fossil beaches.
Diamond Crystals
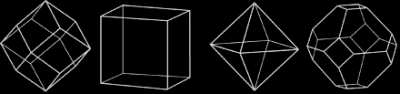
The diamond octahedron has the shape that we describe as a diamond. While it is the most common shape for a diamond crystal, cubes, dodecahedra, and combinations of these three shapes are common. All are highly symmetrical, with equal dimensions in three perpendicular directions, and all are manifestations of the cubic crystal system to which the mineral diamond belongs.
 Exceptions are the flat form called a macle, which is a twin, or composite crystal, as if mirrored across the middle, and etched crystals, with rounded surfaces and, sometimes, elongated shapes. The shapes of diamond crystals can be very intriguing.
Exceptions are the flat form called a macle, which is a twin, or composite crystal, as if mirrored across the middle, and etched crystals, with rounded surfaces and, sometimes, elongated shapes. The shapes of diamond crystals can be very intriguing.
Triangles sometimes result from subtle changes in height on a diamond’s octahedron face and are called trigons.
Hardness
Diamond is renowned for its hardness. Hardness is the measure of a substance’s resistance to being scratched, and only a diamond can scratch another diamond. Diamond is the hardest substance known.
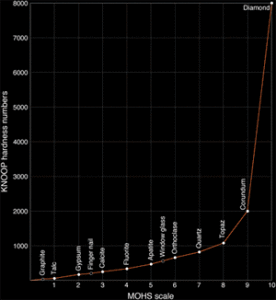 The Mohs scale-a hardness scale developed in 1822 by Austrian Friedreich Mohs as a criterion for mineral identification-can help us appreciate the hardness of diamond. The scale ranks 10 minerals; harder minerals, with a higher number, can scratch those with a lower number.
The Mohs scale-a hardness scale developed in 1822 by Austrian Friedreich Mohs as a criterion for mineral identification-can help us appreciate the hardness of diamond. The scale ranks 10 minerals; harder minerals, with a higher number, can scratch those with a lower number.
When the mineral hardness numbers from the Mohs scale are plotted against those on the more quantitative Knoop scale (based on the force needed to make indentations using a diamond), we can see how it doesn’t adequately express the extreme hardness of diamond. The Mohs scale is relatively stable until it reaches the eighth mineral topaz, but it jumps exponentially from corundum (colorless sapphire) to diamond. It is in fact difficult to measure the hardness of diamond, because diamond must be used to measure its own hardness. A diamond is 58 times harder than the next hardest mineral on earth, corundum, from which rubies and sapphires are formed.
Diamond is the hardest natural substance on earth, but if placed in an oven at 1405 degrees Fahrenheit (763 degrees Celsius), it will vanish. Only a small amount of carbon dioxide will have been released.
It was only during the 15th century when it was discovered that the only way to cut diamonds was with other diamonds. Still, diamonds are brittle; if hit hard with a hammer, they will shatter.
Surface Properties
 How is diamond like a freshly waxed car? It repels water, an unusual property for a mineral. Diamond’s strong bonding and carbon composition cause its surface to repel water but attracts wax and grease. These two properties provide an effective means of separating diamonds from other minerals that come out of mining operations. Washed gravel containing diamonds is flushed with water over a sloping surface covered with a mixture of wax and grease, a “grease table.” The diamonds stick to the table, while the wetted waste minerals wash over it. Gem diamonds readily pick up a greasy film, but cleaning with ammonia or a good detergent restores their brilliance.
How is diamond like a freshly waxed car? It repels water, an unusual property for a mineral. Diamond’s strong bonding and carbon composition cause its surface to repel water but attracts wax and grease. These two properties provide an effective means of separating diamonds from other minerals that come out of mining operations. Washed gravel containing diamonds is flushed with water over a sloping surface covered with a mixture of wax and grease, a “grease table.” The diamonds stick to the table, while the wetted waste minerals wash over it. Gem diamonds readily pick up a greasy film, but cleaning with ammonia or a good detergent restores their brilliance.
Density (Specific Gravity)
Specific gravity deals with the volume of a substance based on its density. In other words, it is how much material is crammed in a specific space. For instance, one pound of lead is much smaller than one pound of water. Specific gravity is the comparison of a certain volume of water to the same volume of another material.
Diamond has a specific gravity of 3.52 which means that a diamond is 3.52 times denser than water. What makes basic carbon so dense? The answer is heat and enormous pressure. Far below the surface of the earth carbon was compressed under intense pressure and heat to cause it to crystallize and form diamonds. The heat was very intense; probably about 1400 Centigrade. But the pressure was even more intense: probably about 300 tons per square inch.
Dispersion
A diamonds brilliance and luster are two of its most valued characteristics. The brilliance of a diamond results from its properties of refraction, reflection, and dispersion.
Upon passing through a diamond facet (one of many small planes cut onto the gem surface), a light ray is refracted, or bent. The bent ray is reflected from a bottom facet upward through a top facet. In refraction each color of the ray is bent at a slightly different angle. This spreading of colors is called dispersion. Since refraction occurs both as the ray enters and as it leaves the diamond, dispersion also occurs twice. Thus the ray is emitted as a glittering rainbow. Of all gems the diamond has the highest index, of refraction: 2.419.
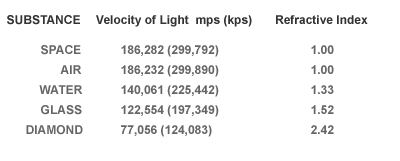
Physics postulates that the speed of light in vacuum is about 186,000 miles per second. The speed or velocity of light is slowed down when it is forced to interact with the electrons of a substance; be it a liquid, gas or solid. In general more dense materials have greater concentrations of electrons and therefore greater contributions to light refraction. Light passing through diamond is reduced to about 77,000 miles per second. Diamond has near the maximum refractive index of any transparent substance.
Reflectance, or the amount of light reflected from a transparent substance, can also be inferred from a material’s refractive index. Diamond displays the maximum amount of reflectance for a transparent substance, displaying what is called an “adamantine” luster.
The refractive index compares the velocity of light in a substance to that in a vacuum. Diamond slows light to a remarkable degree, and ranks high in refractive index.
Electrical Conductivity
Diamonds are basically not very good at conducting electricity. Most are considered semi-conductors meaning that they will only allow a current to pass if there is a great deal of energy trying to get through. Some are even insulators that do allow a current to pass through at all.
However, there is one type of diamond that is a good conductor. That is a Type IIb diamond and it covers mostly the fancy blue diamonds. These are colored by an impurity of boron which makes them conduct electricity as conductors. The importance here is that this can be used to identify natural blue diamonds from treated stones, since the natural blue diamonds will conduct electricity and other diamonds that have been radiated, for instance, will not.
Thermal Conductivity
Thermal conductivity is the ability to transfer heat from one source to another. Diamonds are very, very good at this, which is why there has for years been thermal conductivity testers used to separate diamonds from most imitations such as cubic zirconia. Diamonds are even better than copper in transferring thermal energy which makes them useful in computer and electronic applications to help cool tiny components.
The ability to dissipate heat also one of the reasons diamonds have been called ‘ice’ for many years. Besides looking crystal clear like ice, they transfer heat when touched and thus tend to feel cool to the touch.